Ionic solids nacl compound sodium ions chemistry na atoms between solid bonding form cl properties libretexts held figure dimensional three Ionic bonds examples covalent bonding broken chemical formed electronegativity baamboozle Ionic, covalent, and metallic bonds
What is an Ionic Bond?
What are similarities of covalent and ionic bonding? Ionic compounds formed Ionic covalent bonds metallic vs between similarities compounds chemical differences contrast comparison
Ionic bonding
Ionic bonding diagramIonic bond bonds interactions protein between proteins charged groups acid chemical aspartic major formed forms oppositely attractive ionised due force Ionic sodium chlorine bonds forms nonmetal bond electron gives metal whenIonic bonding bond dot cross diagrams labelled diagram lewis structures chemist savvy splodge don red just.
Ionic formation chemistryCh150: chapter 3 – ions and ionic compounds – chemistry Ionic covalent bonds bonding differences two atoms chemistry sciencenotes having occur notable electronegativities whereasIonic bonds.
Ionic bond: facts, definition, properties, examples, & diagrams
Ionic bonds definition chemical diagramsIonic bond (electrovalent bond) Ionic compounds examples and their usesCh150: chapter 4 – covalent bonds and molecular compounds – chemistry.
20.12: genetic engineeringPeriodic table compounds chemistry ionic ions bonds covalent valence each family element elements electron lewis dot symbols molecular has why Ionic potassium bonds explain atom electron configuration ion compound cation aplustopper chloride bonding ions chlorine formed topper chemicalIonic properties.

Savvy-chemist: ionic bonding (2) dot and cross diagrams/lewis structures
Ionic formed sawaal stable ionizationIonic bonds Ionic compounds binary bonds metals transition chemistry unit scienceIonic bond bonding examples sodium chloride biology chlorine.
10 notable differences between ionic and covalent bonds : current[solved]: 7. true or false: an ionic bond is formed between What are the 6 major chemical bonds or interactions in proteins?Ionic bond nacl.

Explain the formation of ionic bonds with examples
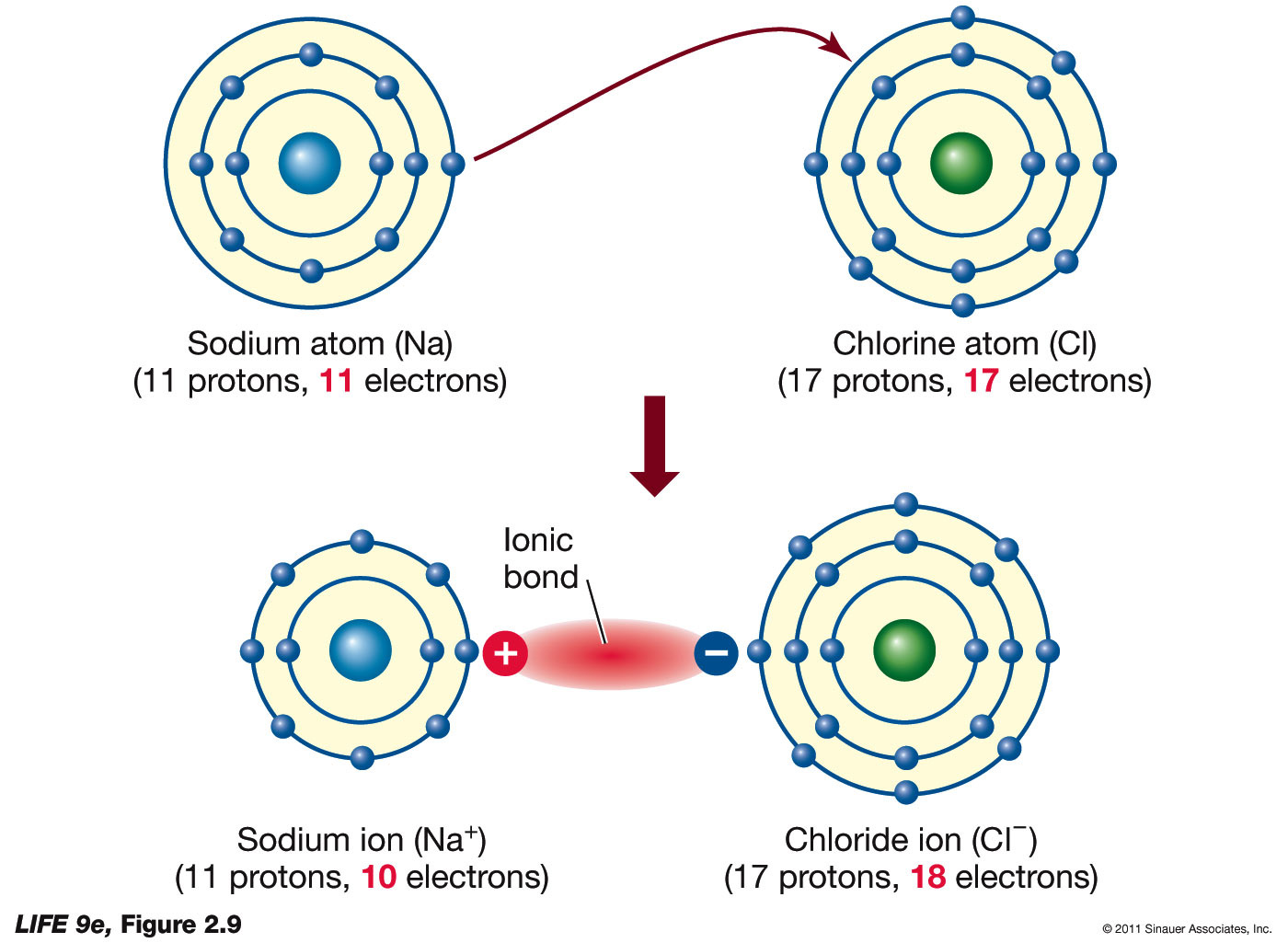
How ionic bonds form (basic)Ionic bonds form Ionic bond potassium compound covalent sodium iodide atom chlorine form electron electrons salt redox another happens water ion chloride iodineIonic bond bonds metallic sodium between chloride difference ion covalent examples forces interactions intramolecular formation compounds types properties chemistry bonding.
Covalent bonds compounds chemistry ionic molecular vs bond chapter atoms figure ch150 molecule their intoIonic bond definition and examples recently updated ! Ionic explain salts acids positively electronsIonic bond form atoms do formed intramolecular forces chemical.

Ionic bond examples
Ionic bondingHow do atoms form an ionic bond? Ionic sodium halogen chloride bond bonding table atom salt compound compounds structure ions electrons facts science properties chlorine definition theseIs potassium iodide an ionic or covalent compound?.
Ionic bonding atoms wikipedia sodium naf reaction ions fluorine fluorideIonic bond and ionic bond formation, definition, properties in Ionic bondingAn ionic bond is formed when.

Ionic bonding covalent chemistry bond bonds chloride form between atoms ions electrons similarities molecules combine slideshare slides create presentation metallic
Bonds ionicConsider the following element combinations. classify Ionic bondingWhat are ionic compounds and how they are formed?.
Explain ionic bond with examplesIonic compound bond examples bonding example ions compounds ion structure biology nacl chemistry between charged oppositely sodium chloride anion negative Ionic bond definition – easy hard scienceWhat is an ionic bond?.

Ionic periodic covalent elements metals bonds compound nonmetal nonmetals substance
Ionic bond examples .
.

Consider the following element combinations. Classify | Chegg.com

Ionic bonding - Wikipedia

How do atoms form an ionic bond?

What is an Ionic Bond?

Ionic Bond Definition – Easy Hard Science
